What it is
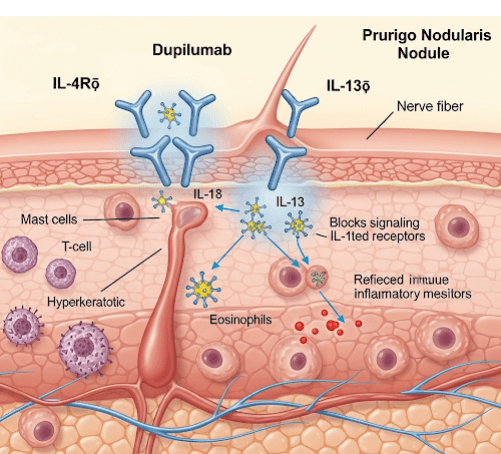
➝ Dupilumab is a fully human monoclonal antibody that blocks the IL-4 receptor alpha (IL-4Rα), thereby inhibiting both IL-4 and IL-13 signaling pathways. These cytokines are central to the Th2-driven inflammation and chronic itch that drive prurigo nodularis (PN).
➝ PN is characterized by severely itchy, firm nodules caused by neuroimmune dysregulation and an itch–scratch cycle.
➝ Dupilumab was recently approved in several countries, including Korea, for moderate-to-severe prurigo nodularis in adults who are candidates for systemic therapy.
Why it’s done
→ To control severe itch, often rated 7–10/10 on itch scales, which disrupts sleep, mood, and daily life.
→ To reduce nodule size, number, and inflammation.
→ To break the itch–scratch cycle, preventing new lesion formation.
→ To improve quality of life, as PN is associated with anxiety, depression, and social isolation.
→ In Korea, dupilumab is considered a major advancement for PN patients who fail conventional therapy (topicals, steroids, phototherapy, immunosuppressants).
Alternatives
→ Topical therapies: corticosteroids under occlusion, calcineurin inhibitors, capsaicin.
→ Intralesional steroids for thick nodules.
→ Systemic immunosuppressants: cyclosporine, methotrexate, azathioprine, thalidomide.
→ Phototherapy: NB-UVB, excimer.
→ Emerging biologics: nemolizumab (IL-31 receptor antibody, clinical trials in Korea), JAK inhibitors (baricitinib, upadacitinib).
Preparation
→ Confirm diagnosis of moderate-to-severe PN with widespread nodules and refractory itch.
→ Assess prior treatment history (failure of topicals, systemic immunosuppressants, or phototherapy).
→ Baseline labs are not routinely required (dupilumab does not need systemic monitoring), but many Korean clinics check CBC, liver/kidney function to rule out coexisting systemic disease.
→ Patient education about injection technique, timeline of improvement, and possible side effects.
How it’s Done
→ Initial dose: 600 mg (two 300 mg subcutaneous injections).
→ Maintenance dose: 300 mg every 2 weeks.
→ Administered subcutaneously in the thigh, abdomen, or upper arm (patient or caregiver can self-inject after training).
→ Treatment is continued long-term; Korean dermatologists often reassess at 16 weeks to determine response.
→ Dupilumab may be combined with emollients, topical steroids, or phototherapy for synergistic effect.
Recovery
→ Clinical trials and Korean real-world reports show rapid itch relief within 2–4 weeks, often before nodules flatten.
→ Significant reduction in nodule number, thickness, and redness by 16 weeks.
→ Many patients achieve complete or near-complete clearance of nodules after several months.
→ Long-term use maintains control with low relapse rates compared to immunosuppressants.
→ Patients report better sleep, mood, and social functioning after itch control.
Complications
→ Generally well tolerated.
→ Most common side effects: conjunctivitis, dry eyes, mild injection site reactions, transient eosinophilia.
→ Rare adverse events: herpes simplex reactivation, arthralgia.
→ No systemic immunosuppression, so safer than cyclosporine or methotrexate for long-term use.
→ In Korea, ophthalmology co-management is common for patients with ocular side effects.
Treatment Options in Korea
→ Dupilumab is available in Korea for moderate-to-severe prurigo nodularis under dermatology prescription.
→ Many tertiary hospitals and specialty dermatology centers are using dupilumab as first-line systemic therapy for PN when conventional treatments fail.
→ Korean clinicians also use dupilumab in PN with atopic dermatitis overlap, which is common in Asian patients.
→ Insurance coverage: Dupilumab is partly reimbursed for PN in Korea under certain severity criteria, making access more feasible.
→ Clinics often integrate dupilumab with digital itch scoring, clinical photography, and patient-reported outcome monitoring to track response.