What it is
➝ Adalimumab is a biologic therapy used for the treatment of moderate-to-severe hidradenitis suppurativa (HS).
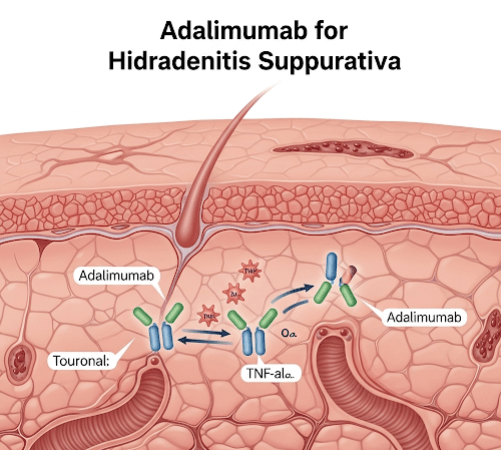
➝ It is a fully human monoclonal antibody that targets tumor necrosis factor-alpha (TNF-α), a key inflammatory cytokine involved in HS.
➝ By blocking TNF-α, adalimumab reduces the chronic inflammation that causes painful nodules, abscesses, and sinus tracts.
➝ It is the first and only biologic drug approved specifically for HS, making it an important treatment option worldwide, including in Korea.
Why it’s done
→ For patients with moderate-to-severe HS who do not respond to antibiotics, hormonal therapy, or surgery alone.
→ To reduce the number of abscesses, nodules, and draining fistulas.
→ To alleviate pain, odor, and pus drainage, which often impact mobility and daily activities.
→ To prevent disease progression and minimize scarring over time.
→ In Korea, adalimumab is considered a key therapy in advanced dermatology centers for HS patients with severe or widespread disease.
Alternatives
→ Medical therapies:
- Oral antibiotics (clindamycin, rifampin, tetracyclines).
- Hormonal therapy (for selected female patients).
- Systemic retinoids (acitretin, isotretinoin in select cases).
→ Other biologics: Clinical studies are exploring IL-17 and IL-23 inhibitors, but these are not yet widely approved for HS.
→ Surgical options:
- Incision and drainage (temporary relief).
- Deroofing (including CO₂ laser deroofing).
- Wide excision for extensive lesions.
→ Lifestyle measures: Weight control, smoking cessation, antibacterial washes.
Preparation
→ Before starting adalimumab, patients undergo screening tests:
- Tuberculosis test (skin test or blood test).
- Hepatitis B and C screening.
- Complete blood count, liver and kidney function tests.
→ Vaccination history is reviewed, as live vaccines cannot be given during therapy.
→ Active infections must be treated before starting adalimumab.
→ In Korea, dermatology clinics provide pre-treatment counseling about injection technique and long-term monitoring.
How it’s Done
→ Adalimumab is given as a subcutaneous injection (under the skin).
→ Dosing schedule for HS:
- Initial dose: 160 mg at week 0 (usually as four injections in one day, or split over two days).
- Second dose: 80 mg at week 2.
- Maintenance dose: 40 mg every week starting at week 4.
→ Injections are usually given in the thigh or abdomen, and patients can be trained to self-administer at home.
→ Korean clinics often use structured injection programs, including nurse-led training and follow-up visits.
Recovery
→ Improvement is often noticeable within 8–12 weeks.
→ Patients may see a reduction in pain, drainage, and new abscess formation.
→ With consistent use, many achieve long-term disease control, fewer flare-ups, and improved mobility.
→ Quality of life significantly improves as patients regain confidence, comfort, and social engagement.
→ However, some patients may require combination therapy with surgery or antibiotics for optimal outcomes.
Complications
→ Common mild side effects: Injection site reactions, headache, upper respiratory infections.
→ More serious risks:
- Increased risk of infections (tuberculosis, fungal, or bacterial).
- Rare cases of lymphoma or other malignancies.
- Possible worsening of demyelinating diseases (e.g., multiple sclerosis).
- Allergic reactions.
→ Regular monitoring is required to detect complications early.
→ In Korea, follow-up usually includes blood tests every 3–6 months and infection screening as needed.
Treatment Options in Korea
→ Adalimumab is widely available in Korean dermatology hospitals and university medical centers, where HS is managed with multidisciplinary care.
→ It is often prescribed as a first-line biologic for patients with stage II–III HS.
→ Korean doctors frequently combine adalimumab with laser deroofing or wide excision surgery, offering a comprehensive approach to reduce recurrence.
→ Clinics emphasize patient education, including self-injection training, safe storage of the medication, and guidance on infection prevention.
→ In Korea, adalimumab is valued as a life-changing therapy for patients with severe HS, offering long-term control and improved quality of life when other treatments fail.